How air pollution causes acid rain
Acid rain, or acid deposition, is a broad term that includes any form of precipitation with acidic components that fall to the ground from the atmosphere in wet or dry forms. How can rain be acidic, and what are the effects of acid rain? What is being done to prevent acid rain? Read on more to find out.
What is acid rain?
Acid rain is rain that has been made acidic by certain pollutants in the air. It is a type of acid downfall, which can appear in forms such as wet depositions (rain, sleet, and snow) and dry depositions (gasses and dust particles). Both wet and dry deposition can be carried by wind, sometimes for very long distances. Acid rain can fall on buildings, cars, and trees, and can make lakes acidic. In addition, acid rain can also be inhaled by people and cause health problems.
What causes acid rain?
Acid rain results when sulfur dioxide (SO2) and nitrogen oxides (NOx) are emitted into the atmosphere and transported by wind and air currents. SO2 and NOx react with water, oxygen and other chemicals to form sulfuric and nitric acids. These then mix with water and other materials before seeping into the ground, causing harmful effects.
The main source of SO2 and NOx causing acid rain comes from burning of fossil fuels for energy generation: Two thirds of SO2 and one fourth of NOx in the atmosphere can be traced to electric power generators. In addition, exhaust from cars, trucks, and buses also release NOx and SO2 into the air and cause acid rain.
The image below illustrates the acid rain pathway in our environment in the following order:
- Emissions of SO2 and NOx are released into the atmosphere, where
- the air pollutants are then transformed into acid particles that may transport in long distances.
- These acid particles fall to the earth as wet and dry depositions and
- as a result cause harmful effects on soil, forests, lakes, and other environments.
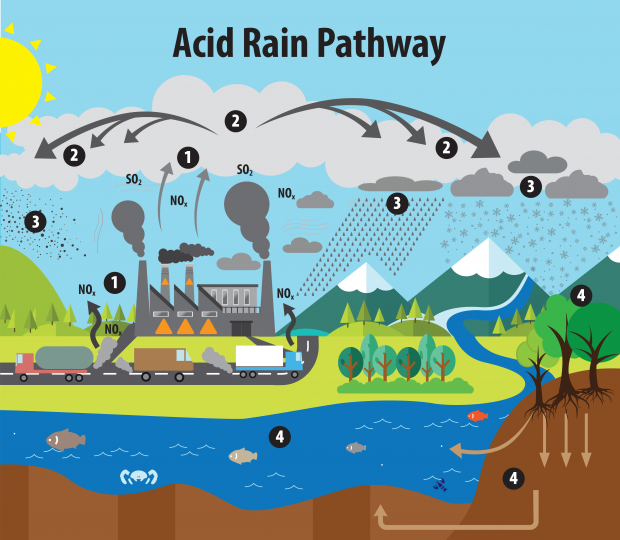
The acid rain pathway. Image source: US EPA
What makes rain so acidic?
Pure water has a pH value of 7.0 (neutral); however, natural, unpolluted rainwater is already acidic by default with a a pH value of about 5.6. The acidity of rainwater comes from the natural presence of three substances found in the troposphere (the lower layer of the atmosphere): carbon dioxide (CO2), nitric oxide (NO), and sulphur dioxide (SO2). CO2 is present in the greatest concentration and steadily increasing, given that the average value has been greater than 400 ppm over the last five years. CO2 is therefore the main contributor to the natural acidity of rainwater.
Acid rain is caused by reactions in the environment, and although some rain is naturally acidic with a pH level of as low as 5.0, human activities have made it worse. Normal precipitation — the collection of water molecules that fall from the sky as rain, sleet, hail, or snow — reacts with naturally-occurring alkaline chemicals or non-acidic materials that then reacts to neutralize natural acids. However, if the precipitation becomes too acidic (for instance through man-made influences), then these materials may not be able to neutralize all of the acids. The resulting acidification of the ground results in damage to crops, trees, lakes, rivers, and animals.
Why is acid rain harmful?
Acid rain triggers a number of inorganic and biochemical reactions which are now becoming a growing, worldwide environmental problem. Many lakes have become so acidic that fishes and other living organisms cannot thrive any longer. Important soil minerals can also be lost due to acid rain washing them away: One such example is Ca2+ (calcium), a mineral required for the creation of roots, leaves and other organic matter, wose absence thus can kill trees and damages crops. Additionally, the degradation of soil minerals also releases toxic ions such as Al3+ (aluminium) into the water supply, making it undrinkable and unsustainable.
Acid rain can also damage stones in two primary ways: dissolution and alteration. Depending on the composition, some stones are more susceptible to acidic deposition than others. When the SO2 and NOx react with calcite in marble and limestone, the calcite dissolves. This can be shown in buildings with roughened surfaces, removal of material, and loss of carved details. This is one of the ways how air pollution can damage even the built environment.
Finally, acid rain can have a negative impact on human health: The reaction of SO2 and NOx in the atmosphere forms sulfate and nitrate particles that can be inhaled and cause certain health impacts. A study has shown that exposures to sulfate particles and particulate matter pollution were associated with people at risk for cardiovascular diseases having their heart rate reduced. However, the study still notes that there should be further investigations conducted in order to understand the health risks better.
What can be done about acid rain?
Government agencies, scientists, and even normal citizens can do their part to reduce acid rain. It is important to first understand the problem and its root causes, as this makes a difference in educating citizens about taking the right steps:
The government limits the amount of SO2 that power plants can release into the air through policy, together with their NO emissions.. They can also set strategic goals to focus on more efficient and environmentally-friendly ways to produce energy without the use of fossil fuels. These energy sources, such as solar and wind power, can help reduce the production of acid rain as these methods produce less pollution.
Scientists have also found various ways to reduce the amount of SO2 that originates from coal-burning power plants: using coal that contain less sulfur, “washing” coal so that it produces less sulfur or installing power plant equipment called “scrubbers”, which remove the SO2 from gasses leaving the smokestack. The burning of coal and fossil fuels must be changed in order to reduce SO2 and also NOx, which are created during the burning process.
Citizens can also do their part to help reduce emissions! Cars and trucks are a huge factor that adds to the pollution in the air. Carpooling, taking public transportation, and using a bike are some ways to minimize the miles. Citizens can conserve energy in a number of ways that limits blue light exposure from electronic devices, controlling the temperature at home when needed, and limiting air conditioning. At Breeze Technologies, citizens also have an opportunity to become an air quality sensor host and monitor the air in the vicinity of their neighborhood! Contact us to become an active protector of the air today!



 Unsplash.com / sporlab
Unsplash.com / sporlab